You need hardware you can trust for your business. The Muha Meds 2g Disposable is special because each batch has paperwork. You can also see models with reports kept on file. This is only about hardware like the shell, atomizer, battery, airway, and packaging. It does not include oils, new muha flavors 2025, or health.
Key Takeaways
-
Always make sure your Muha Meds devices follow rules like UN 38.3 and RoHS. This helps your business stay safe and follow the law. - Keep all your papers neat and easy to find. This lets you show proof fast during checks or audits. - Check QR codes and batch numbers to see if your devices are real. This stops you from using fake products.
Compliance & Documentation
Regulatory Standards Overview
You need to check if your empty vape devices follow the rules. The Muha Meds 2g Disposable must meet some important standards. These rules help with shipping, safety, and legal needs. Here are the main ones you should know:
|
Standard |
Description |
Key Points |
|---|---|---|
|
UN 38.3 |
This rule is for moving lithium batteries safely. |
It makes sure batteries are packed right and have paperwork. |
|
RoHS |
This rule limits bad substances in electronics. |
It keeps ten harmful things out to protect people and nature. |
|
IEC 62133-2 |
This rule is about safe rechargeable lithium batteries. |
It checks for electrical safety and stops short circuits. |
|
UL 8139 |
This rule is for vaping device safety. |
It looks at fire risks and electric shock; certified ones get a special label. |
|
REACH/SVHC |
This rule is about chemicals in products sold in the EU. |
It says you must tell customers if a product has too much of a bad chemical |
These rules do not cover oils or new muha flavors 2025. They only focus on hardware like the battery, shell, and atomizer. You can ask for reports for each batch or model. You can get these reports from approved sellers.
Neutral Language in Compliance Claims
Do not make promises you cannot prove. Use careful words when you talk about following rules. For example, say "reports available upon request" instead of "certified safe." This keeps your claims true and protects your business.
Some common compliance problems are:
|
Compliance Issue |
Description |
|---|---|
|
Jurisdiction first |
Only buy, fill, and sell in places where it is legal. |
|
Hardware vs. filled goods |
Empty shells are not the same as finished cannabis products; each needs its own approval. |
|
Shipping & transport |
Lithium batteries have special shipping rules; suppliers should give you UN 38.3 test papers. |
|
Cells & protections |
You need paperwork about battery chemistry and safety circuits. |
|
UN 38.3 |
Devices with built-in batteries need a UN 38.3 test paper for shipping. |
|
IEC design references |
Following safety rules helps stop problems in the field. |
|
Storage & handling |
Devices must be shipped and stored at the right temperatures. |
Tip: Always ask for the newest batch reports and keep them safe. If you want to fill devices with new muha flavors 2025, make sure your hardware paperwork is current.
Device Selection & Specs
Muha Meds 2g Disposable Features
You can pick how much air you want to pull in. If you want a tighter draw, you get warmer and thicker vapor. If you want more air, the vapor feels cooler and lighter. Micro-seal technology helps stop leaks. This keeps your product safe when you move or store it. Each device has a QR code and batch number. You can scan them to see lab reports and paperwork.
The device has built-in safety features. It protects against overcharge, over-discharge, short-circuit, and heat. The USB-C port lets you charge it quickly. An indicator light shows if the device is ready. The package is tamper-evident and child-resistant. This helps you follow safety rules for licensed fillers. All these features make the Muha Meds 2g Disposable a good choice for new muha flavors 2025.
Device Models & Specs Table
You may want to compare different device models. The table below shows the main specs for the Muha Meds 2g Disposable and other empty devices. This helps you choose the best hardware for your needs.
|
Model |
Capacity |
Airway/Resistance |
Oil Inlet / Atomizer Material |
Rechargeability / Interface |
Protection Features |
Packaging Traceability |
|---|---|---|---|---|---|---|
|
Muha Meds 2g Disposable |
2g |
Adjustable (tight/open) |
Porous ceramic / Food-grade steel |
USB-C rechargeable |
Overcharge, over-discharge, short, thermal protection |
QR code, batch number, tamper-evident, child-resistant |
|
Comparable Model A |
1g |
Fixed (medium) |
Ceramic / Stainless steel |
Micro-USB rechargeable |
Overcharge, short protection |
Batch code, tamper-evident |
|
Comparable Model B |
2g |
Fixed (open) |
Ceramic / Alloy |
USB-C rechargeable |
Overcharge, over-discharge protection |
QR code, batch number |
This table helps you see what makes the Muha Meds 2g Disposable special. It has adjustable airflow, strong safety features, and easy tracking. These things help you get ready for new muha flavors 2025 and keep your paperwork in order.
Verification & Traceability
Five-Step Verification Process
You want to make sure every Muha Meds empty device you receive is real and safe for your licensed filler operation. Here’s a simple five-step process you can follow:
-
Check the outer packaging. Look for any damage, tampering, or missing seals. Genuine packaging should look clean and professional.
-
Inspect print and seals. Examine the print quality, logos, and security seals. The text should be sharp, and seals should not show signs of being opened.
-
Verify QR code, batch, and serial numbers. Make sure the QR code, batch number, and serial number all match the paperwork and the device itself.
-
Confirm the authorized channel. Only accept shipments from your approved supplier list. Double-check the company name, tax ID, and contact details.
-
Handle exceptions. If you spot anything odd, take a photo, keep the product aside, and contact support. Do not use or fill questionable devices.
Tip: Following these steps helps you avoid counterfeits and keeps your documentation in order.
QR Code & Batch Number Checks
Muha Meds uses QR codes and batch numbers to help you confirm device authenticity. You can find a verification sticker on the box. Scan the QR code with your phone. If the code matches the batch and leads to the official Muha Meds site, you know the device is genuine. If the code does not match or the website looks suspicious, the device may be fake. Batch numbers also help you trace each device back to its test reports and paperwork.
-
QR codes make it easy to check if your device is real.
-
Batch numbers link your device to compliance documents.
-
If you find a mismatch, do not use the device and report it to your supplier.
Requesting Documentation
When you need paperwork for a batch or device, you should contact sales or support. Make sure you include all the details they need to find the right documents. Here’s what you should provide:
|
Required Field |
Description |
|---|---|
|
Model Name |
Example: Muha Meds 2g Disposable |
|
Batch Number |
Printed on the box or device |
|
Purchase Order Number |
Your order reference |
|
Serial Number/Photo |
Clear photo of the serial or QR code |
|
Date of Purchase |
When you received the shipment |
|
Supplier Name |
Who you bought it from |
Note: Always keep copies of your requests and the documents you receive. This helps you stay ready for audits or shipping checks.
Sourcing & Operations
Authorized Channel Checks
You need to buy Muha Meds empty devices from trusted sellers. First, check the company name and their business or tax ID. Make sure they have a real address and a working email or phone. Many good sellers use a ticket system for support. Reliable suppliers deliver on time and answer quickly. They also get high review scores. They should have ISO 9001 certification. They must share lab reports from other companies.
|
Indicator |
Description |
|---|---|
|
On-time delivery rates |
Try to find sellers with delivery rates above 95%. This helps you avoid supply problems. |
|
Response times |
Good sellers reply in less than 6 hours. This means they talk to you fast. |
|
Review scores |
Look for ratings above 4.5 out of 5 from trusted business sites. This shows they are reliable. |
|
ISO certifications |
Make sure your supplier has ISO 9001 certification. This proves they care about quality. |
|
Quality verification processes |
Ask for lab test reports and factory audit papers. These show the products are checked. |
B2B Terms & Inspection Checklist
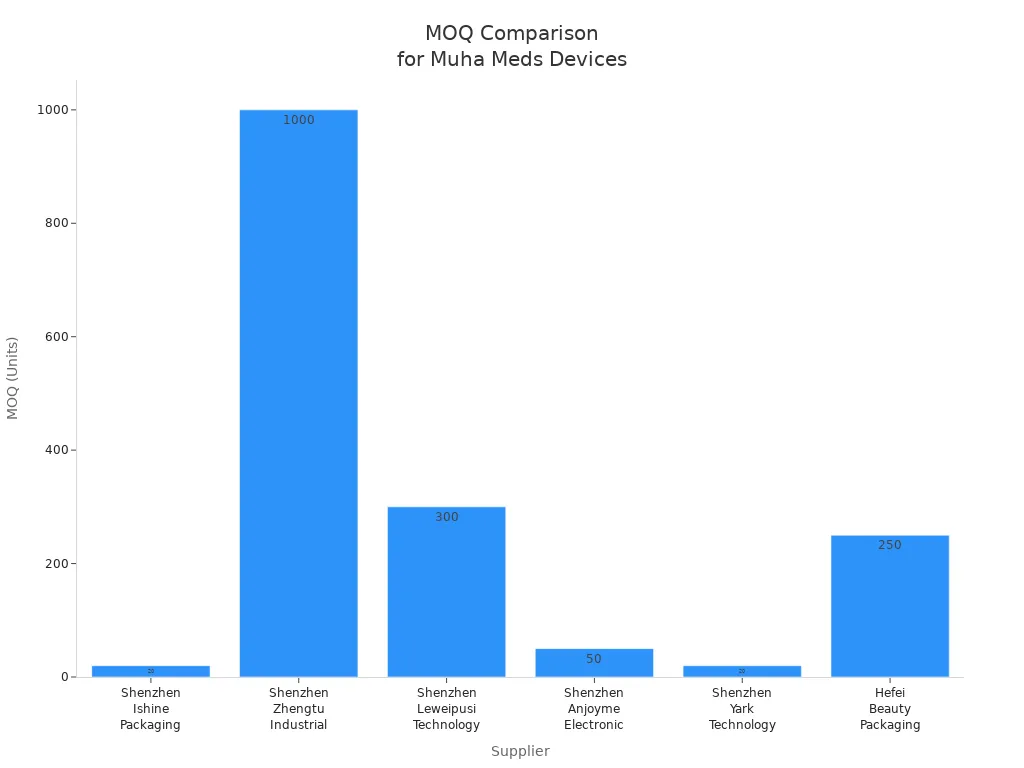
When you order empty devices, you need to know the business rules. These rules include minimum order quantity, lead time, payment type, Incoterms, warranty, return policy, and service agreement. Here is a table that shows how many units different suppliers ask for:
|
Supplier |
MOQ (Units) |
|---|---|
|
Shenzhen Ishine Packaging Co., Ltd. |
20 |
|
Shenzhen Zhengtu Industrial Co., Ltd. |
1000 |
|
Shenzhen Leweipusi Technology Co., Ltd. |
300 |
|
Shenzhen Anjoyme Electronic Technology Co., Limited |
50 |
|
Shenzhen Yark Technology Co., Ltd. |
20 |
|
Hefei Beauty Packaging Technology Co., Ltd |
250 |
示意/需以实际报告为准.
When your shipment comes, use this checklist to make sure everything is okay:
-
Scan the QR code. It should work and go to the right website.
-
Look for a California sticker if you sell there.
-
Check the logo. Make sure there are no spelling mistakes.
-
Compare prices. If the price is much lower than usual, be careful.
-
Check the box quality. Boxes should be strong and colors should look right.
-
Make sure the seller has a license.
-
Match the packing list and serial numbers.
-
Take pictures of random units for your records.
-
Write down the temperature, humidity, and any damage.
-
Keep all compliance papers for at least 36 months.
UL 8139 checks electrical, heating, charging, and battery safety for vaping devices. Always ask for a real UL 8139 report for each hardware model.
Technical Dimensions
Airway & Structure
When picking empty devices for licensed filling, you should check the airway and structure. The Muha Meds 2g Disposable lets you change the resistance. You can make the draw tighter or looser by changing the air inlet size. The airway length changes how much the vapor cools before you breathe it in. Inside, there is a system that controls condensation and backflow. This stops liquid from leaking into the airway, so your product stays safe.
-
Key points to check:
-
Adjustable or fixed resistance
-
Air inlet size and placement
-
Airway length for vapor cooling
-
Condensation and backflow control (mechanism only)
-
Atomizer Unit
The atomizer unit is the most important part of the device. Muha Meds uses a porous ceramic coil in their atomizer. This coil stays wet and spreads heat evenly. You get steady vapor and fewer dry hits. The atomizer can also warm up gently before use. This helps the vapor come out smooth and even.
Here’s how different atomizer specs affect performance and compliance:
|
Specification |
Impact on Performance and Compliance |
|---|---|
|
Coil Resistance |
Affects heating efficiency and vapor production |
|
Materials Used |
Influences flavor preservation and safety compliance |
|
Design Features |
Determines compatibility and device reliability |
Battery & Transport
You need to check the battery and if it is ready for shipping. The Muha Meds 2g Disposable has a USB-C rechargeable battery. It meets UN 38.3 rules for shipping lithium batteries. The box and label must show battery warnings and batch numbers. This helps you pass shipping checks and keeps your paperwork organized.
|
Specification |
Details |
|---|---|
|
Thread Type |
510-thread |
|
Voltage Range |
3.2V - 3.7 |
Always ask for the newest UN 38.3 test report. Make sure the packaging matches the paperwork. This keeps your licensed filler operation safe and following the rules.
Versioning & Evidence Management
Version Log
You need to keep a clear record of every change made to your Muha Meds empty device batches. A version log helps you track updates, spot issues, and show proof during audits. You can use a simple table to organize this information. Here’s a sample version log you might use:
|
Version |
Change Summary |
Responsible Person |
Date (UTC) |
Related Report ID |
|---|---|---|---|---|
|
v1.0 |
Initial release |
J. Smith |
2024-12-01 |
SGS-3825 |
|
v1.1 |
Updated battery protection |
L. Chen |
2024-12-15 |
UL-8139-25 |
|
v1.2 |
Improved airway seal |
M. Patel |
2025-01-05 |
INT-2025R |
Keep your version log up to date. This makes it easy to match each device batch with its test reports and paperwork.
Internal QC Methods
You want every batch to meet your standards. Internal quality control (QC) helps you catch problems early. Here are some methods you can use:
-
Sampling: Pick random units from each batch for testing. This helps you spot defects before filling.
-
Environment Checks: Record temperature and humidity in your storage and filling areas. Devices work best in the right conditions.
-
Puff Curve & Weighing: Use a puff machine to check airflow and resistance. Weigh devices before and after simulated use to confirm no leaks.
You can keep a QC record like this:
|
Batch Number |
Sample Size |
Test Date |
Inspector |
Result Summary |
Linked Report ID |
|---|---|---|---|---|---|
|
MM2025A |
20 |
2025-01-10 |
K. Lee |
Pass |
SGS-3825 |
Tip: Keep all QC records and link them to your version log and evidence registry. This helps you stay ready for audits and keeps your operation smooth.
If you choose Muha Meds empty devices with the right paperwork, your licensed filling job gets easier and safer. You will find it simple to use, with many strains to pick from, steady dosing, and lab-checked quality:
|
Benefit |
Description |
|---|---|
|
Convenience |
Easy to use and simple to take with you |
|
Variety of Strains |
Many options for everyone |
|
Consistent Dosing |
You get the same amount every time |
|
Lab-Tested Quality |
Tested by labs and clear reports |
Always check your devices in steps, buy from trusted sellers, and keep your records organized. This blog only talks about hardware and paperwork. Rules can change by location, so always check your local laws. If you need reports or want to check if a seller is approved, contact sales or support.
FAQ
How do you verify Muha Meds empty device documentation?
You scan the QR code and check the batch number. Ask your supplier for the latest test reports. Always match paperwork to your device.
What standards should your empty devices meet for licensed filling?
Your devices should meet UN 38.3, RoHS, IEC 62133-2, UL 8139, and REACH/SVHC. These standards help you ship, store, and fill devices safely.
Can you request compliance reports for each batch?
Yes, you can. Contact sales or support with your batch number and order details. They will send you the correct documents.


























0 Comments